Abstract
Relapsed or refractory acute myeloid leukemia (AML) patients have scarce treatment options despite the recent emergence of novel targeted therapies. Chimeric antigen receptor (CAR) T-cell therapies have been approved for the treatment of B-cell malignancies and multiple myeloma, but it remains very challenging to treat myeloid malignancies with this therapeutic modality, in part due to the absence of specific tumor antigens in these indications. Antigens expressed on AML cells are usually shared with healthy hematopoietic stem/progenitor cells (HSPC) and targeting them can lead to life-threatening on-target/off-tumor toxicity. CD84 antigen is an immunoreceptor member of the SLAM family (SLAMF5), whose expression has been reported in certain immune cells and in B-cell malignancies. In this study, we show for the first time that CD84 is overexpressed in AML and present the first results of CD84-targeting CAR T-cells for the treatment of this disease.
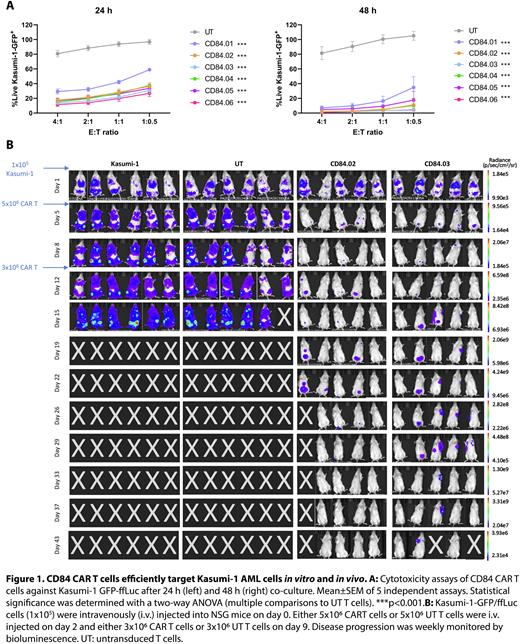
We analyzed AML primary samples and found that CD84 is overexpressed on the surface of AML cells, both de novo and at relapse, while lower expression was detected in CD34+ HSPC. Furthermore, immunohistochemistry analysis of healthy human tissues showed that CD84 is not expressed in the parenchyma of liver, kidney, lung, myocardium, skin, or brain (cerebral cortex and cerebellum), although expression was detected in tissue monocytes/macrophages. This expression pattern is similar to that of CD123, a clinical stage and safe CAR T target in AML. Anti-CD84 CAR T cells were engineered using both fully human and murine CD84-binding sequences. The sequences of the variable regions of the light (VL) and the heavy (VH) chain were used to design different single-chain variable fragments (scFv), changing the order (VLVH or vice versa) and the linker length. In total, we designed 21 lentiviral second-generation CARs with an anti-CD84 scFv, a CD8α hinge and transmembrane domain, a 4-1BB costimulatory and a CD3ζ signaling domain. Some constructs were discarded due to failed lentiviral transduction or target recognition; the following CAR T-cells were characterized in depth: one with a fully human scFv, CD84.01 (VHVL), and 5 with different murine scFv, CD84.02 (VLVH), CD84.03 (VHVL), CD84.04 (VHVL), CD84.05 (VHVL), and CD84.06 (VHVL). All these CAR T-cells displayed cytotoxic activity towards, and were able to proliferate and secrete cytokines in vitro when exposed to the CD84high Kasumi-1 and the CD84low U937 AML cell lines (Figure 1A). We then analyzed their cytotoxicity against monocytes, B cells and T cells purified from the same donor as the CD84 CAR T cells were generated; all CART cells exerted some cytotoxic activity against their own T cells, which was much lower than that against Kasumi-1 cells. To assess the myeloablative potential of CD84 CAR T cells, we carried out cytotoxic assays against CD34+ HSPCs purified from 1) donor cord blood; 2) directly from donor bone marrow samples, or 3) an apheresis product of a donor for an allogeneic hematopoietic stem cell transplant. We found that the cytotoxic effect against CD34+ HSPC was much lower than that against leukemic cells. Based on these in vitro results, the CARTs CD84.02 and CD84.03 were tested for their efficacy in a human NSG xenograft mouse model of AML (Kasumi-1). Although CD84.02 appeared to be more effective than CD84.03, both were able to control the disease for at least 43 days (Figure 1B), at which point no leukemic cells were detected in the bone marrow and spleen of CAR T-treated animals. In contrast, CD84 CAR T cells were found in these organs at the end of the experiment.
In summary, here we report that CART cells directed against CD84 can eliminate AML cells both in vitro and in vivo. Our data support the therapeutic use of CD84 CAR T cells for relapsed/refractory (R/R) AML. However, due to its potential myelotoxicity, we encourage its first use in a clinical trial as a bridge therapy to allogeneic stem cell transplant for patients with R/R AML.
Disclosures
Pérez-Amill:Gyala Therapeutics S.L.: Current Employment. Armand-Ugon:Gyala Therapeutics S.L.: Current Employment. Peña:Gyala Therapeutics S.L.: Current Employment. Val Casals:Gyala Therapeutics S.L.: Current Employment. Santos:Gyala Therapeutics S.L.: Current Employment, Current holder of stock options in a privately-held company. Urbano-Ispizua:Miltenyi: Consultancy; Celgene: Consultancy; Gilead: Consultancy. Juan:Gyala Therapeutics S.L.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal